Introduction
Difference Between Enantiomers and Diastereomers - Welcome to the world of stereochemistry, where molecules assume distinct three-dimensional configurations, giving rise to the fascinating world of stereoisomers. In this article, our focus turns to unraveling the distinctions that set apart Enantiomers and Diastereomers. If you're curious about how these isomers differ and what sets them apart, you're in the right place. With the expertise and authority that we bring, you'll gain a profound understanding of this captivating topic.
 |
| Difference Between Enantiomers and Diastereomers |
Enantiomer Vs Diastereomers
|
Characteristic |
Enantiomers |
Diastereomers |
|
Relationship |
Mirror images of each other,
non-superimposable |
Not mirror images, can be
superimposable in some cases |
|
Chirality |
Always chiral (R and S
configurations) |
May or may not be chiral |
|
Optical Activity |
Equal and opposite optical
activities (dextrorotary and levorotary) |
May have different or no optical
activities |
|
Chemical Properties |
Identical chemical properties,
challenging to separate |
Different chemical properties, can
be separated more easily |
|
Occurrence |
Common in nature, e.g., amino
acids in proteins |
Less common in nature |
Enantiomers and diastereomers
Enantiomers and diastereomers are two fundamental categories of stereoisomers. Enantiomers are mirror-image isomers, meaning they are non-superimposable and have the same physical and chemical properties except for their ability to rotate plane-polarized light in opposite directions. Diastereomers, on the other hand, are stereoisomers that do not share this mirror-image relationship and possess distinct physical and chemical properties, making them easily differentiable. This differentiation is particularly important in the field of organic chemistry, where understanding these isomeric relationships is crucial for various applications, such as drug development and biochemistry.
Enantiomers and diastereomers Example
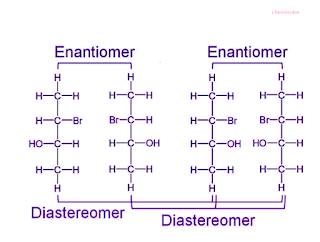 |
| Enantiomers and diastereomers |
Superimposable mirror images
Superimposable mirror images refer to a unique property found in enantiomers, a class of stereoisomers. Enantiomers are molecules that possess a non-superimposable mirror-image relationship, meaning that while they have the same molecular composition and connectivity, they cannot be perfectly overlaid onto one another. This characteristic arises from their chirality, where one enantiomer is the mirror image of the other, much like a right and left hand. This property has significant implications, particularly in fields like pharmacology and biochemistry, where even subtle differences in the spatial arrangement of atoms can have profound effects on a molecule's interactions with other chiral compounds, such as enzymes and receptors.
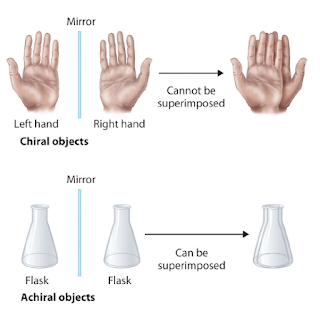 |
| Superimposable mirror images |
Difference Between Enantiomers and Diastereomers
Stereoisomers are isomeric molecules that have the same molecular formula and connectivity but differ in their spatial arrangement. Within this category, we have two distinct types: enantiomers and diastereomers. Let's explore how they differ:
Enantiomers
Enantiomers are molecules that are non-superimposable mirror images of each other. This means that if you were to hold one enantiomer up to a mirror, it would appear as the other enantiomer. These molecules share the same physical and chemical properties, except for one crucial aspect - their interaction with plane-polarized light.
Chirality of Enantiomers
Enantiomers are chiral, which means they have a "handedness." One is considered "right-handed," while the other is "left-handed." This property is described using the terms R and S in organic chemistry.
Optical Activity of Enantiomers
The key differentiator is their optical activity. One enantiomer rotates plane-polarized light clockwise (dextrorotary), while the other rotates it counterclockwise (levorotary). This property is utilized in chiral analysis.
Chemical Properties of Enantiomers
Enantiomers have identical chemical properties, making them challenging to separate through traditional chemical reactions.
Diastereomers
In contrast to enantiomers, diastereomers are stereoisomers that are not mirror images of each other. They exhibit different physical and chemical properties, making them easier to distinguish.
Chirality of Diastereomers
Diastereomers may or may not be chiral. Their dissimilarity arises from differences in the spatial arrangement of atoms, rather than a mirror-image relationship.
Optical Activity of Diastereomers
Unlike enantiomers, diastereomers do not cancel each other's optical activities when mixed in equal proportions.
Chemical Properties of Diastereomers
Diastereomers have distinct chemical properties due to their unique spatial arrangements. This dissimilarity makes it easier to separate them using chemical reactions.
FAQs
Are enantiomers and diastereomers the only types of stereoisomers?
No, there are other types of stereoisomers, such as cis-trans isomers, which occur in compounds with restricted rotation around a double bond.
Can enantiomers and diastereomers have the same physical properties?
Enantiomers share most physical properties, but diastereomers have different physical properties due to their unique arrangements.
Why is chirality important in chemistry?
Chirality is crucial because it affects how molecules interact with other chiral compounds, such as enzymes and receptors. It has significant implications in drug development and biochemistry.
How can one distinguish between enantiomers and diastereomers?
Enantiomers can only be distinguished through their optical activity, while diastereomers have different physical and chemical properties.
Can diastereomers have more than two stereoisomers?
Yes, diastereomers can have multiple stereoisomers because they result from variations in multiple chiral centers or geometric isomerism.
Are enantiomers commonly found in nature?
Yes, enantiomers are prevalent in nature. For example, the amino acids in proteins are enantiomers.
Conclusion
In the captivating world of stereochemistry, the Difference Between Enantiomers and Diastereomers lies in their mirror image relationship and distinct properties. Enantiomers are mirror image twins with opposing optical activities, while diastereomers exhibit different physical and chemical properties. Understanding these isomeric relationships is crucial in various fields, from pharmaceuticals to biochemistry. We hope this article has shed light on the fascinating world of stereoisomers, providing you with valuable insights and expertise.




